closed
Antonella Marano, Federico II University of Naples, Italy
3-years Project
Celiac disease
Area: Immunology
Grant: 002/2019
- Title: Characterization of TcRγδ+ IELs in the progression from potential to active celiac disease
- Duration: Triennial Project
- Principal Investigator: Antonella Marano, Federico II University of Naples, Italy
- Tutor (Head Lab): Prof. Mariantonia Maglio, Federico II University of Naples, Italy
Publications originating from the Project
- Marano A, Troncone R, Discepolo V, Maglio M. Combined RNAscope and immunohistochemistry staining on duodenal paraffin sections as a new tool to reveal cytolytic potential of intraepithelial lymphocytes. J Immunol Methods. 2023 Jun;517:113470. doi: 10.1016/j.jim.2023.113470. Epub 2023 Apr 8. PMID: 37037412. https://pubmed.ncbi.nlm.nih.gov/37037412/
- Marano A. et al. ‘Characterization of TcRγδ+ and TcRαβ+CD8+ intraepithelial lymphocytes in the natural history of celiac disease’. In preparation.
- Marano A. et al. ‘Predictive biomarkers in a large cohort of potential celiac disease patients: a longitudinal study’. In preparation.
THE STUDY
Project rationale and aims
Celiac disease (CD) is characterized by an infiltration of the duodenal mucosa by both TcRαβ+ and TcRγδ+ intraepithelial lymphocytes (IELs). While TcRaβ+CD8+ IELs expressing the activating natural killer receptors (NKRs) NKG2D and NKG2C/CD94 are the main responsible for tissue destruction in active CD (ACD), the function of TcRγδ+ IELs in CD pathogenesis remains unclear. The CD spectrum includes potential CD (PCD) patients, who show an adaptive immune response to gluten with positive serum anti-tissue transglutaminase and a normal (Marsh 0) or only slightly infiltrated (Marsh 1) duodenal mucosa. Among them, some evolve toward ACD while others remain PCD for years. Thus, PCD represent a unique model to investigate pathogenetic events preceding tissue destruction.
We postulated that the expansion and functional activation of a subset of TcRγδ+ IELs might contribute to full-blown CD. The rationale behind this hypothesis lay in the observation that in ACD TcRγδ+ IELs carrying the Vδ1+ chain lost the expression of NKp46/NKp44 receptors, in contrast to non-CD controls (CTR). Importantly, those Vδ1+ IELs abundant in ACD produced IFN-γ, suggesting that they might exert pro-inflammatory functions (Mayassi et al. Cell 2019). Concomitantly, the small intestinal epithelium of ACD patients showed a loss of butyrophilin-like8 (BTNL8) expression, a molecule that has a role in TcRγδ+ cell biology.
To better understand TcRγδ+ IELs/epithelial cells interaction and their contribution to CD progression, we investigated their phenotype in comparison to TcRaβ+CD8+ IELs in different stages of CD. Furthermore, we studied the epithelial compartment aiming at defining the mechanisms leading to TcRγδ+ IELs recruitment, expansion and activation.
The characterization of these cell subsets might help identifying biomarkers useful to predict, among PCD patients, who will develop tissue damage.
Research plan and results obtained
First of all, we evaluated by Immunohistochemistry (IHC) the density of IELs in the small intestinal epithelium of CD patients and to better characterize IELs in CD patients, we evaluated the expression of NKRs, such as NKG2A and NKp46, in CD45+CD3+CD8+ and CD45+CD3+TcRγδ+VD1+ IELs by flow cytometry. Our data showed that PCD is a heterogeneous group of patients: a subset expressing ACD-like features, another with CTR-like features with respect to NKRs. On the other hand, the density of activating NKG2D+ IELs was detected by IHC, showing no increase in expression, like controls.
In order to investigate the functional features of IELs, we looked at their cytotoxic properties by evaluating the density of Perforin (PFN)+ IELs stained by IHC. We observed an increase of Perforin (PFN)+ IELs in a subgroup of PCD. In order to better characterize those IELs, we developed a double assay that combines RNAscope and IHC. The former was used to reveal PFN transcripts, while IHC to detect TcRγδ+ or CD8+ and discriminate the two IELs subsets on duodenal sections of ACD and both M0- and M1-PCD patients.
RNAscope technique is able to detect single mRNA transcripts of target protein with a double-Z strategy that allows to amplify the target signal. The combined RNAscope-IHC assay was set up for the first time (Marano A. et al. J Immunol Methods, 2023) and enabled us to visualize two markers, PFN and CD8 or TcRγδ IELs, on the same cell in the intestinal biopsies of CD patients (Figure 1).
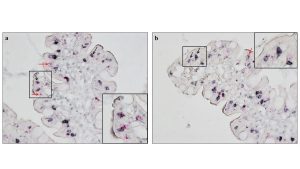
Figure 1. Combined RNAscope and IHC to identify perforin positive IELs. PFN positive TcRγδ+ (a) or CD8+IELs (b) in the duodenal epithelium of PCD patients are visualized as black round cells with red dots within the cytoplasm. In both images black arrows point at double positive cells and red arrows point at perforin single positive cells. Magnification 400x.
Finally, we investigated the expression of the following markers in the small intestinal epithelium and lamina propria by IHC: BTNL8, IL-15, IL-21, MxA and HLA-E. With respect with the epithelial expression of those markers, we also observed a dichotomy among PCD patients, suggesting that possibly epithelial stress recapitulates the heterogeneity observed when analysing NKRs expression in distinct IELs populations.
Experimental design and methodologies
Taking advantage of a large cohort of PCD patients available in our institute, we used duodenal biopsies collected from those patients along with those from CTR subjects, GFD and ACD patients, to retrospectively study the small intestinal epithelial compartment and IELs in relation to the progression of CD by IHC on either formalin-fixed paraffin-embedded (FFPE) or optimal cutting temperature (OCT) compound-embedded duodenal biopsies. We analyzed the following markers: CD3, TcRγδ, PFN, NKG2D, HLA-E, BTNL8, MxA, IL-15, IL-21. Furthermore, PFN positive cells were assessed by RNA-scope, an in-situ hybridization technology that allows to detect mRNA on FFPE tissue, in combination with IHC to highlight CD8 and TcRγδ positive IELs.
We prospectively recruited non-CD CTR and CD patients (PCD, ACD and GFD) to carry out flow cytometry experiments. IELs were freshly isolated from duodenal biopsies and processed to evaluate the surface expression of inhibiting NKG2A and NKp46 NKRs in CD45+CD3+CD8+ or CD45+CD3+TcRγδ+VD1+ IELs (Figure 2).

Figure 2. Schematic of study design. Summary of methods and biological samples used for retrospective and prospective parts of the study, in the left and right frames respectively.
Potential pitfalls and caveats
Most difficulties encountered during the project were related to the SARS-CoV-2 pandemic. These include delays in receiving laboratory supplies as well as a significant reduction of the number of EGDS procedures performed in our Paediatric Hospital, that impacted patients’ recruitment. As a consequence, during the first year, we focused on the retrospective part of the study. Nevertheless, the slow recovery of clinical activities allowed us in the second year to recruit patients undergoing upper endoscopy, whose biopsies were used to perform flow cytometry experiments on freshly isolated IELs.
Conclusions and discussion
Overall, this study allowed to improve our knowledge regarding the immune response in celiac disease, particularly with the regard to lymphocytes involved in intestinal damage. Our data seem to support the concept that PCD is a heterogeneous group of patients, especially with regard to the epithelial compartment, including both IELs and enterocytes.
As summarized above, our data showed for the first time that PCD patients can be divided in two groups based on the NKRs asset on IELs: an ACD-like and a CTR-like subset with respect ot the NKRs profile.
On this basis, we speculate that ACD-like PCD group could develop full-blown disease, while the other one could remain PCD. Later studies will define whether the first group is more prone to develop villous atrophy over time.
Moreover, we developed a new technique for the investigation of the cytolytic features of IELs subgroups by performing the combined RNAscope-IHC assay on duodenal sections of CD patients. If further validated in future studies, this technique may be able to improve the diagnosis in doubtful or difficult cases, such as in PCD patients. This technique allowed us to show that PCD with no global IELs infiltration (M0-PCD) display a cytolytic phenotype, in contrast to those with an increased infiltration (M1-PCD).
These data suggest that TcRγδ+ IELs displayed cytolytic properties at the early stage of PCD, but loose them over the disease course. We hypothesize that a change in TcRγδ+IELs function, might be crucial in the progression toward overt-CD.
In addition, we observed that some inflammatory changes persist mainly in the intestinal epithelium of GFD patients, suggesting that some typical signs of the disease persist despite gluten being excluded from the diet. The significance of these changes is still unclear.
