closed
Vera Rotondi Aufiero, ISA-CNR, Avellino, Italy
Triennial Fellowship
Celiac Disease
Area: Immunology
- Grant: FC 014/2017
- Title: Immunomodulatory function of γδ+ intraepithelial lymphocytes in Celiac Disease: in vivo and in vitro responses to gliadin stimuli
- Duration: Triennial Project
- Principal Investigator: Vera Rotondi Aufiero, Istituto di Scienze dell’Alimentazione, ISA-CNR, Avellino, Italy
- Tutor (Head Lab): Giuseppe Mazzarella, Istituto di Scienze dell’Alimentazione, ISA-CNR, Avellino, Italy
Publications
Project rationale and aims
Celiac disease (CD) is a serious autoimmune disease that occurs in genetically predisposed people where the ingestion of gluten leads to damage in the small intestine. Increased number of intraepithelial lymphocytes (IELs), crypth hyperplasia, and villous atrophy are the histomorphological hallmark of CD (Jabri et al, 2009).
In humans, the intraepithelial lymphocytes (IELs) are mainly CD8+ T cells expressing the αβ T-cell receptor (TCR) as well as T cells expressing the γδ TCR (Chang et al, 2005). In active CD, both these types of IELs increase in density.
Gluten withdrawal, has a different impact on IELs. In treated CD patients, the numbers of gluten-specific CD4+ T cells in lamina propria and CD8+ αβ TCR IELs rapidly decrease, whereas γδ+ IELs remain elevated (Calleja et al, 2011).
In treated CD, γδ+ IELs, could play a prevalent regulatory role as showed, in vitro, by the secretion of TGF-β (Bhagat et al, 2008). Despite the increased frequency in vivo and suppressive activity in vitro, γδ+ IELs do not control the development of inflammation in the small intestinal mucosa of active CD, suggesting a defect in the activation of regulatory mechanisms (Bhagat et al, 2008).
It is known that IL-15 is overexpressed in the epithelial compartment from active CD (Iacomino et al, 2016). It was previously reported (Maiuri et al, 2001) that an over-expression of IL-15 in organ culture of treated CD patients and in a transgenic mouse model (Inagaki-Ohara et al, 1997), led to an expansion of γδ+ IELs. Moreover, the Cerf-Bensussan group showed that IL-15 was involved in the local downregulation of TGF-β signalling (Benhamed et al 2007). In addition, our group showed that IL-15 impairs the functions of T regulatory (Treg) cells in CD (Zanzi et al 2011).
Taken together these data indicate that IL-15 is an important player in CD pathogenesis ,affecting the role of γδ+ IELs in active CD.
Among all the techniques used to analyze cytokines pathways of a specific cell population, laser capture microdissection (LCM) allowed a cell type-specific molecular analysis of solid tissues, without contamination from surrounding cells, and provided new insights into both normal cellular biology and pathogenic mechanisms.
The aim of this study was to investigate the cytokine expression profile of ʏδ+ IELs isolated from intestinal mucosa of active CD patients, by LCM. Moreover, by using the organ culture model of intestinal biopsies from active CD patients (Mazzarella et al, 2003; Iacomino et al. 2021) we investigated, in vitro, the role of IL-15 in modulating the mucosal immune response .
Research plan and results obtained
TASK 1: By immuno-LCM on serial specular sections we analysed γδ+ IELs-specific cytokine production in the intestinal mucosa of active CD patients with Marsh II and Marsh III lesions.
Results of TASK 1:
Anti-inflammatory response of γδ+ IELs.
γδ+ IELs from Marsh II CD patients showed a statistically significant increase (p<0.05) of IL-10 compared to γδ+ IELs isolated from Marsh III patients. On the contrary, γδ+ IELs isolated from Marsh III CD patients retained a statistically significant (p<0.05) increase of TGF-β production compared to γδ+ IELs isolated from Marsh II CD patients (Figure1) .
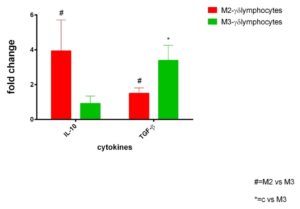
Figure 1. Anti-inflammatory response from γδ+ IELs in active CD patients: IL-10 and TGF-β mRNA transcripts analysed by RT-qPCR in γδ+ intraepithelial lymphocytes isolated by immuno-LCM in jejunal biopsies from active celiac disease patients on Marsh II and Marsh III degrees (n =10 for each group ) . Fold change (y axis) represents messenger RNA expression normalized to GAPDH. mRNA expression was normalized with control epithelium of each patient. Differences associated with statistical significance are marked by respective symbols. # p<0.05
Inflammatory response of γδ+ IELs
γδ+ IELs, isolated by immuno-LCM, from CD patients with Marsh III lesion showed a higher expression of IL-17, IFN-γ and TNF-α pro-inflammatory cytokines than γδ+ IELs isolated from intestinal mucosa of CD patients with Marsh II lesion(Figure2).
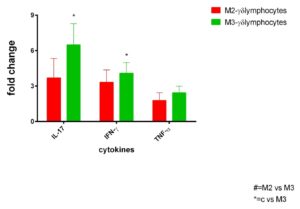
Figure 2. Inflammatory cytokines from γδ+ IELs from active CD patients. Selected mRNA transcripts analysed by RT-qPCR in γδ+ intraepithelial lymphocytes (IELs) isolated by immuno-LCM in jejunal biopsies from active celiac disease patients on Marsh II and Marsh III degrees (n =10 for each group ) . Fold change (y axis) represents messenger RNA expression normalized to GAPDH. mRNA expression was normalized with control epithelium of each patient. No differences associated with statistical significance are present.
TASK 2: By using the organ culture model, we analysed if IL-15 could influence the production of pro-inflammatory and anti-inflammatory cytokines.
Results of TASK 2:
Jejunal biopsies from active CD patients were cultured for 24 h with or without IL-15 neutralizing antibody.
After 24h of culture, we analysed the production of IFN-γ pro-inflammatory cytokine and anti-inflammatory TGF-β, by RT-qPCR.
We demonstrated that anti-IL-15 Ab was effective to prevent the local downregulation of TGF-β as well as to downregulate IFN-γ secretion (Figure 3).
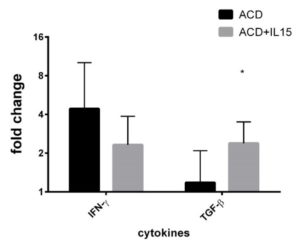
Figure 3. Effect of anti IL15 antibody in active CD. Selected mRNA transcripts analysed by RT-qPCR in whole jejunal biopsies from active celiac disease patients (n =9) in colture for 24 h with and without an anti IL15 antibody . Fold change (y axis) represents messenger RNA expression normalized to GAPDH. Solid lines referred to intergroup (IELs vs IEs ) differences associated with statistical significance are marked by respective symbols. *p<0.05).
Experimental design and methodologies
EXPERIMENTAL DESIGN:
Task 1: Anti-inflammatory (IL-10 and TGF-β) and pro-inflammatory (IL-17, TNF-α and IFN-γ) cytokines production from γδ+ IELs, isolated by immuno-LCM on serial specular sections from intestinal biopsies of active CD subjects with different histological types of lesion (Marsh II and Marsh III).
Task 2: The effects of the neutralizing action of IL-15 on the production IFN-γ and TGF-β, were evaluated in small intestinal biopsies obtained from active CD patients cultured with and without IL-15.
METHODOLOGIES:
TASK 1 : IMMUNO-LCM ON SERIAL SPECULAR SECTIONS
Active CD patients on histological stages Marsh II (n.10) and Marsh III (n. 10) were recruited in Avellino Area. Freshly frozen jejunal mucosa samples were mounted on the cryostat stage (Leica CM1850; Leica Microsystems, Wetzlar, Germany) and sectioned at 8 µm. The mirror sections were obtained by two consecutive sections, mounted on two different glass slides. In particular, the first section, mounted on a typical glass slide, was immunostained for γδ+ T cells as previously reported (Maglio et al, 2017). The consecutive section was mounted upside- down on an RNase-free membrane slide (PEN-membrane, 1 mm glass, Carl Zeiss MicroImaging, Munich, Germany) so that the same tissue cut surface was exposed like the first one, and processed for LCM.
TASK 2: ORGAN CULTURE
Jeujenal biopsies from 9 active CD patients were challenged for 24h in organ culture, with and without an anti IL-15 antibody, to investigate if IL-15 could interfere with the expression of cytokines involved in CD pathogensis.
TASK 1 and 2:
RNA EXTRACTION and RETROTRASCRIPTION
Total RNA was obtained from microdissected samples or cryostatic sections from each cultured biopsy, by PicoPure RNA isolation kit according to the manufacturer’s protocol (Arcturus, Life Technologies).Isolated RNAs were reverse transcribed using the SuperScript VILO cDNA Synthesis Kit (Life Technologies).
RT-qPCR and Statistical analysis
IL-10, TGF-β, IL-17, IFN-γ ,TNF-α, IL-21 and IL-15 cDNAs were quantified by SYBR green (PowerSYBR Green PCR Master Mix, Applied Biosystems) -based qRT-PCR assays on an ABIPRISM 7000 SDS instrument (Applied Biosystems). Gene expression was normalized to the level of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Relative expression of transcripts was calculated by the ΔΔCt method using the Data Assist Software v3.01 (Applied Biosystems). Statistical analyses were performed with GraphPad Prism (GraphPad Software, CA, USA). Results were reported as normalized mean expression. Differences between groups were compared using unpaired two-tailed Student’s t-tests. Statistical significance was achieved when p < 0.05.
Potential pitfalls and caveats
TASK 1: The expression profile of the main cytokines produced by γδ+ IELs from active CD, isolated by immuno-LCM on serial specular sections was possible only in active CD patients on Marsh III and II. In fact, only by such patients we were able to collect a number of γδ+ IELs (about 1000 cells) cells, within the limits of 30 minutes necessary for LCM, such as to extract an amount of RNA which was useful for downstream molecular analyses.
TASK 2: As we have not been able to isolate γδ+ IELs by LCM, we evaluated the effect of anti IL-15 on the whole active CD jejunal biopsies, cultured with and without the neutralizing anti IL-15 antibody .
We analysed the neutralizing effect of IL15 on the whole biopsies of active CD patients after 24 h of culture, evaluating the expression of the main proinflammatory and anti-inflammatory cytokines involved in active CD.
Conclusions and discussion
TASK 1: γδ+ IELs isolated from Marsh II or Marsh III CD patients produced both pro-inflammatory and anti-inflammatory cytokines. Particularly, we showed a higher production of IL-10 from γδ+ IELs in Marsh II compared to Marsh III and a higher production of TGF-β in Marsh III than Marsh II.
Moreover, γδ+ IELs isolated from both Marsh II and Marsh III patients producedIL-17, IFN-γ and TNF-α , with a higher expression in Marsh III compared to Marsh II.
In conclusion, our data suggest:
- γδ+ IELs isolated from active CD patients retain an anti-inflammatory activity;
- Th-1/Th-17 immune response is correlated with the inflammatory status.
- γδ+ IELs in active CD patients exhibit functional plasticity, secreting both pro- and anti-inflammatory cytokines.
Recent data have shown the presence of TCR αβ+ gliadin-specific CD4 + IL-17-producing T cells in the duodenal lamina propria of active CD patients, that simultaneously produce TGF-β (Fernandez et al, 2011), suggesting a functional plasticity of such cells.
Several studies have established a functional plasticity of γδ+ T cells, ruled by different environmental factors, as specific cytokines, growing factors, inflammation mediators, in physiological and pathological conditions (Paul et al, 2014).
Recently, it was shown in active CD, that chronic site-specific inflammation permanently reconfigures the tissue-resident γδ+ IEL compartment in Th1-like phenotype characterized by production of IFN-γ (Mayassi et al, 2019).
Further investigations are needed to define if we are in presence of a single subset of γδ+ IELs, that switch from anti- to pro-inflammatory activity or two distinct subsets with different cytokine producing capacities and functions, which could by induced by different environmental factors.
TASK 2: We demonstrate that the anti IL-15 Ab was able to upregulate TGB-β production and downregulate IFN-γ in CD patients.
On the basis of these results and on the finding that the function of Treg may be substantially limited by IL-15 (Zanzi et al,2011), we suggest that therapies aimed to neutralize such cytokines may not only decrease bystander T-cell activation but also reconstitute the suppressor function of T reg cells.
Such, hypothesis deserve additional investigation.
Moreover, future work will be necessary to improve the immuno-LCM protocol aimed to isolate γδ+ IELs after organ culture.
However, the results obtained in this study improve our understanding about the role of γδ+ IELs and IL-15 in CD pathogenesis .
References
– Benahmed M, Meresse B, Arnulf B, Barbe U, Mention JJ, Verkarre V, Allez M, Cellier C, Hermine O, Cerf-Bensussan N. Inhibition of TGF-beta signaling by IL-15: a new role for IL-15 in the loss of immune homeostasis in celiacdisease. Gastroenterology. 2007 Mar;132(3):994-1008. doi: 10.1053/j.gastro.2006.12.025. Epub 2006 Dec 16. PMID: 17324400.
– Fernandez, I.J.Molina, P.Romero, R.Gonza´lez, J.Pen˜a, F.Sa´nchez, F.R. Reynoso, J.L.Pe´rez-Navero, O.Estevez, C.Ortega, M.Santamarı´a. Characterization of gliadin-specific Th17 cells from the mucosa of celiacdiseasepatients. Am J Gastroenterology
– Mayassi T, Ladell K, Gudjonson H, McLaren JE, Shaw DG, Tran MT, Rokicka JJ, Lawrence I, Grenier JC, van Unen V, Ciszewski C, Dimaano M, Sayegh HE, Kumar V, Wijmenga C, Green PHR, Gokhale R, Jericho H, Semrad CE, Guandalini S, Dinner AR, Kupfer SS, Reid HH, Barreiro LB, Rossjohn J, Price DA, Jabri B. Chronic Inflammation Permanently Reshapes Tissue-Resident Immunity in Celiac Disease. Cell. (2019), Feb 21;176(5):967-981.e19. doi: 10.1016/j.cell.2018.12.039. Epub 2019 Feb 7. PMID: 30739797; PMCID: PMC6667191
– Paul, A.K.Singh, G.Shilpi, Lal. Phenotypic and functional plasticity of gamma-delta (γδ) T cells in inflammation and tolerance. Int Rev Immunol. (2014), Nov-Dec;33(6):537-58. doi:10.3109/08830185.2013.863306. Epub 2013 Dec 19. PMID: 24354324
– Zanzi D, Stefanile R, Santagata S, Iaffaldano L, Iaquinto G, Giardullo N, Lania G, Vigliano I, Vera AR, Ferrara K, Auricchio S, Troncone R, Mazzarella G. IL-15 interferes with suppressiveactivity of intestinalregulatory T cellsexpanded in Celiacdisease. Am J Gastroenterol. 2011 Jul;106(7):1308-17. doi: 10.1038/ajg.2011.80. Epub 2011 Apr 5. PMID: 21468011.
