closed
Albino Coglianese, University of Salerno, Italy
Triennial Fellowship
Celiac Disease
Area: Epidemiology
Grant: 011/2018
- Title: Definition and validation of biomarkers to evaluate Gluten Free Diet (GFD) compliance in celiac patients.
- Duration: Triennial Project
- Principal Investigator: Albino Coglianese, University of Salerno, Italy
- Tutor (Head Lab): Prof. Viviana Izzo, University of Salerno, Italy
Publications originating from the Project
- Coglianese A, Charlier B, Mensitieri F, Filippelli A, Izzo V, Dal Piaz F. Standard addition method (SAM) in LC-MS/MS to quantify gluten-derived metabolites in urine samples. J Pharm Biomed Anal. 2023 Apr 24;232:115416. doi: 10.1016/j.jpba.2023.115416. Epub ahead of print. PMID: 37120973. https://pubmed.ncbi.nlm.nih.gov/37120973/
THE STUDY
Project rationale and aims
Nowadays the only treatment available for celiac disease (CD) is the gluten-free diet (GFD). A tight adherence to a gluten-free diet is essential to reduce symptoms, avoid nutritional deficiencies and improve patients quality of life. Current guidelines recommend, for both symptomatic and asymptomatic patients, periodic monitoring of GFD adherence. To date, the methods available to monitor adherence to GFD in CD patients are not sensitive enough to detect occasional dietary transgressions, which may cause damage to the intestinal mucosa. In many cases, adherence to a GFD is evaluated by subjective and unreliable measures, such as questionnaires; these latter, besides the intrinsic difficulties of standardization, time and costs, have significant limitations, such as the reliance on memory capacity of the patients and their actual truthfulness in reporting information. As a consequence there is undoubtedly the need for more reliable and reproducible methods. The alkylresorcinols (ARs) and their main metabolites, 3,5-dihydroxybenzoic acid (DHBA) and 3-(3,5-dihydroxyphenyl)-propanoic acid (DHPPA), have been proposed as biomarkers for the intake of whole wheat and rye. High levels of these phenolic lipids were detected in the serum and urine of celiac patients, with a substantial decrease following a GFD. Therefore, the main focus of the present research was to develop and validate an analytical method for the simultaneous detection of these two biomarkers of interest in urine samples, thus correlating the different concentrations of the two biomarkers to GFD adherence.
Research plan and results obtained
The method developed in this project was fully validated according to the most recent guidelines. We found that this method allows to quantify the two urinary metabolites with good accuracy and precision. The lower limit of quantification (LLOQ) was evaluated and defined as the lowest analyte concentration that can be measured with acceptable accuracy (bias% ±15%) and precision (CV% ≤15%). As shown in figure 1 (A), LLOQ for DHBA and DHPPA are 100 and 150 ng/mL, respectively. To investigate the occurrence of matrix-induced variability, matrix effect (ME) and extraction recovery (ER) were evaluated on 6 different urine samples. Values considered acceptable are between 60 – 140 % and within ±15% for ER and ME, respectively. Both DHBA and DHPPA showed a satisfactory ER and ME in all urine samples tested (Figure 1 B). The intraday and interday repeatability were evaluated by repeating the analysis 5 times on the same day and in 5 non-consecutive days, respectively. Repeatability fulfil the acceptable criteria (bias% ±15%, CV% ≤ 15%) (Figure 1 C). Analytes stability was evaluated in the urine sample. Stability is acceptable (percentage of degradation (%D) ≤ 15%) at all times and all storage conditions tested for both DHBA and DHPPA, except for the 30 day storage (Figure 1 D).
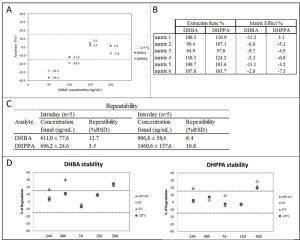
Figure 1. Results of method validation. (A) DHBA and DHPPA lower limits of quantification (LLOQ). (B) Extraction rate and matrix effect of DHBA and DHPPA in six urinary matrices. (C) Repeatability interday and intraday of DHBA and DHPPA. (D) Urine sample stability of DHBA and DHPPA.
Once the method has been fully validated, we analyzed urine samples, collected from three healthy volunteers, subjected to a gluten challenge (Figure 2). In the first three days a gluten-free diet was followed, on the third day 100 gr of white bread were assumed (gluten intake). Successively, following a further day of gluten-free diet, a gluten based diet was restored. Metabolite levels were observed by morning urine analysis on each collection day. A substantial trend in the urinary levels of the biomarkers of interest in presence and / or absence of gluten was observed. These evidences, even though were obtained on a small number of analyses, support the potential use of DHBA and DHPPA as biomarkers for gluten intake.

Figure 2. In A) the dietary regimen and the expected levels of urinary metabolites are shown. In B) concentration, expressed in ng/mL, and in C) the trend of the concentration of biomarkers (DHBA and DHPPA) during the diet period.
Experimental design and methodologies
In this project, we developed and validated a quantification method, using UHPLC–MS/MS for the dosage of two urinary metabolites of ARs, DHBA and DHPPA (Figure 3). Stable isotopes of DHBA and DHPPA were used as internal standard (IS), to counterbalance procedural errors and variations in instrumental response. Compounds extraction was achieved by liquid-liquid extraction (LLE) and their quantification by a standard addition method (SAM) application. Our SAM approach used a three-point calibration and a urine volume less than 1 mL per sample, greatly reducing sample volume needed. The chromatographic method optimized involves the use of a hydrophilic interaction liquid chromatography (HILIC) in a direct phase approach. The use of a UHPLC-MS/MS, considered a gold standard technique for quantitative analysis, allowed to achieve the good reproducibility and sensitivity needed for the detection of traces of endogenous substances within complex matrices such as urine.
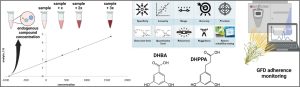
Figure 3. Graphical representation of the experimental design and methodologies applied in this project.
Potential pitfalls and caveats
Several drawbacks have been encountered during the development of the present project. From a methodological point of view, the definition of the correct extraction and chromatographic separation protocol was fundamental in order to minimize the matrix effect. The presence of these metabolites also in other dietary sources, although too low to preclude the use of these metabolites as biomarkers of gluten intake, poses an important problem to the set-up, development and validation of a suitable methodological approach. After trying different quantification strategies (background subtraction, surrogate matrix approach) to solve this problem we adopted the SAM method.
Conclusions and discussion
In this project, we developed a SAM quantification method, using UHPLC–MS/MS for the dosage of two urinary metabolites of ARs, DHBA and DHPPA selected as potential biomarkers for short-term monitoring of GFD adherence in celiac patients. The method, fully validated, allowed to quantify urine metabolites with good sensitivity, accuracy and reliability. In addition, we carried out a proof of concept of the validated method by quantifying metabolites levels in healthy volunteers. Data of these preliminary analyses showed an overall accordance in biomarkers “trend” among different subjects (figure 2). The small gluten intake derived from bread was responsible for a significant raise in biomarkers levels, on day 4. DHBA seems to be more sensitive in revealing small variations in gluten assumption. Moreover, it is worth noting that when a full gluten rich diet is restored at day 5, biomarkers levels significantly raise their concentration at day 6 morning urines. In general, it is possible to observe that great variations in biomarkers levels are evident between a GFD and a gluten rich one. These preliminary evidences might help establishing a threshold for gluten unvoluntary sudden intake, when a large cohort of patients may be analysed. Furthermore, this application helped us to understand if our method allowed the quantification of even small biomarkers concentrations and slight variations in their concentrations after intake. Clearly, these data were not considered as clinical evidence or evaluation of biomarkers trend in the population, since only a small number of samples from healthy volunteers in two dietary regimens were considered. However, the method proved to be able to successfully discriminate metabolites levels in the urine of subjects undergoing different diets. A clinical study will be needed to further investigate the biomarkers potential in correlate for gluten intake and in defining a possible “threshold” level, below which the presence of biomarkers can be considered in line with a gluten free diet.
