closed
Lucia TrePpiccione, National Council for Research, Avellino, Italy
Triennial Fellowship
Celiac disease
Area: Immunology
Grant: FC 006/2018
- Title: Down-regulation of Immunogenic and cytotoxic properties of gliadin through its enzymatic transamidation or via the activation of the Nrf2 pathway.
- Duration: Triennial Project
- Principal Investigator: Lucia Treppiccione, Istituto di Scienze dell’Alimentazione, ISA-CNR, Avellino, Italy
- Tutor (Head Lab): Dr. Mauro Rossi, Istituto di Scienze dell’Alimentazione, ISA-CNR, Avellino, Italy
Publications originating from the Project
- Treppiccione L, Picarelli A, Rossi M. Beneficial Role of Microbial Transglutaminase in the Pathogenetic Mechanisms of Coeliac Disease. J Pediatr Gastroenterol Nutr. 2022 Jun 1;74(6):728-733. doi: 10.1097/MPG.0000000000003451. Epub 2022 Apr 19. PMID: 35442226. https://pubmed.ncbi.nlm.nih.gov/35442226/
- Treppiccione L, Luongo D, Maurano F, Rossi M. Next generation strategies to recover immunological tolerance in celiac disease. Int Rev Immunol. 2023;42(3):237-245. doi: 10.1080/08830185.2022.2044807. Epub 2022 Feb 28. PMID: 35225129. https://pubmed.ncbi.nlm.nih.gov/35225129/
- Treppiccione L, Maurano F, Rossi S, Luongo D, Rossi M. Transamidated wheat gliadin induces differential antigen recognition in the small intestine of HLA/DQ8 transgenic mice. Food Funct. 2022 Aug 30;13(17):8941-8950. doi: 10.1039/d2fo02032g. PMID: 35929785. https://pubmed.ncbi.nlm.nih.gov/35929785/
Project rationale and aims
Celiac disease (CD) is a food intolerance caused by an immune reaction to wheat gluten and related proteins of barley and rye. Gluten-derived peptides cause the duodenal lesion in CD patients, following their tissue-transglutaminase mediated deamidation, by generating a T-cell mediated inflammatory response. Previous studies reported that wheat gliadin following transamidation by microbial transglutaminase (mTG) does not induce IFN-y secretion by intestinal cells from CD patients.
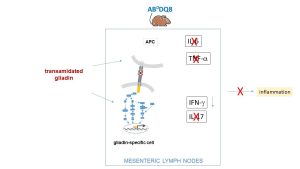
Figure 1 – In DQ8 mice, the production of pro-inflammatory cytokines is significantly reduced in the presence of modified gladin.
The scope of this project was to analyze furtherly the immune activity of spf, in particular as an inducer of tolerance to gliadin.
To address the issue, we initially studied the recall response of splenic T cells from DQ8-tg mice to gliadin and spf in vitro. After this analysis, we then explored possible tolerization protocols, using spf as antigen.
Research plan and results obtained
To analyze the mechanisms underlying immunomodulatory activity, we tested initially the recall response of splenic T cells specific to gliadin and spf in vitro in DQ8-tg mice and found that the immune response in spleen cells derived from spf immunized mice was lower than in cells from gliadin immunized mice. Furtherly, we confirmed the inability of transamidated gliadin to reproduce the IFN-y levels induced by native gliadin.
Then, we determined timing and antigen doses for mucosal immunization in DQ8 mice by intranasal administration of pt-gliadin along with cholera toxin (CT) as a mucosal adjuvant. We found that after 49 days mice were significantly sensibilised.
Next, different tolerisation protocols were studied in this model:
- A first experimental protocol was based on suckling DQ8 mice. Phosphate-buffered saline, pt-gliadin or pt-spf were given intragastrically, to mothers. When offspring was 6-8 weeks old, it was intranasally sensitized. On day 49, mice were sacrificed to analyse spleen and MLNs cells. The results indicated that the either gliadin or spf administration in lactating mothers did not down-regulate the gliadin-specifc response in offspring.
- A second protocol was based on tolerogen co-administration during antigen stimulation in adult mice. 6-week-old mice were treated intragastrically with pt-gliadin or pt-spf. One day a week they received intranasally pt-gliadin + CT. On day 49, mice were sacrificed and spleen and MLNs cells were analyzed. The results reported confirmed that spf did not induce an inflammatory response in vitro. However, co-treatment did not induce tolerance to gliadin.
- A third protocol was based on tolerogen pre-treatment. 6-week old mice received intragastrically pt-gliadin or pt-spf. On day 22, all animals were mucosally sesnitised. On day 49, mice were sacrificed and spleen and MLNs cells were analyzed, The results indicated that pretreatment with spf down-regulated the INF-g response in splenic cells but not in MLN cells. Moreover, results indicated that tolerance induction was not associated to an increase of IL-10 levels.
Experimental design and methodologies
A-PAGE: Acid-polyacrylamide gel electrophoresis was used to characterize the gliadin profiles.
MULTIPLEX ANALYSIS EMD Millipore’s MILLIPLEX map Mouse Cytokine/Chemokine Magnetic Bead kit was used on selected samples for the simultaneous quantification of 32 analytes (G-CSF, GN-CSF, IFN-γ, IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-9, IL-10, IL-12 (p40), IL-12 (p70), IL-13, IL-15, IL-17, IP-10, KC, LIF, LIX, MCP-1, M-CSF, MIG, MIP-1α, MIP-1β, MIP-2, RANTES, TNF-α and VEGF. Result analysis is still ongoing).
MUCOSAL IMMUNIZATION: Mouse on a gluten-free diet, were mucosally immunised by nasal administration in multiple doses of native gliadins plus cholera toxin (CT) On day 49, mice were sacrificed to collect mesenteric lymph node (MLN) and spleen. MLN cells and splenic cells were isolated and in vitro challenged with both antigens; after 72 h supernatants were assayed by ELISA or Multiplex.
TOLERIZATION PROTOCOL: Three experimental protocols were tested as described above. The protocol on suckling mice was adapeted from Turfkruyer et al Muc Imm 2016;9:479 and Verhasselt et al, Nature Medicine, 14(2),170-175.
Potential pitfalls and caveats
A limitation of this work is associated with the biological variability and the maximum number of animals to be used for each experiment. Nevertheless, we obtained statistically differences that support our results.
Another intrinsic difficulty is the ability to induce gliadin-specific immune tolerance in sensitised mice. Tolerance toward gluten remains difficult to generate not only in individuals but also in animal models. There are many questions about the mechanisms that contribute to break mucosal tolerance to gluten in CD. Nevertheless, in our work, we obtained a systemic tolerance in DQ8 tg mice by pre-treatment with spf, thus demonstrating the immunomodulatory property of this modified gliadin.
Conclusions and discussion
Results showed the ability of spf to revert the classical inflammatory response Interestingly, our results also showed a systemic gluten tolerance in DQ8 mice after pre-treatment of spf. Therefore, an innovative approach to recover gluten tolerance has been highlighted, based on the use of modified antigen molecules that encourages and supports the use of spf to restore oral tolerance to gluten.
However, further investigations are necessary to find the right protocol for our purpose, that is the mucosal tolerisation to gliadin, and with a view to using modified gluten as prophylaxis for patients genetically predisposed to CD.
