Giorgia Renga, University of Perugia, Italy
Triennial Fellowship
Celiac disease
Area: Inflammation
Grant: FC 008/2018
- Title: Exploiting the mast cells plasticity for predictive signatures of Candida albicans commensalism vs pathogenicity in celiac disease.
- Duration: Triennial Project
- Principal Investigator: Giorgia Renga, University of Perugia, Italy
- Tutor (Head Lab): Prof. Luigina Romani, University of Perugia, Italy
- Collaborations: Dr. Valeria Villella, European Institute for Research in Cystic Fibrosis (IERFC), San Raffaele Hospital, Milan, Italy; INMI IRCCS L. Spallanzani, Rome, Italy.
Publications originating from the Project
Project rationale and aims
Celiac disease (CD) is an autoimmune enteropathy resulting from an interaction between diet, genome, and immunity. Whilst many patients respond to a gluten free diet, in a substantive number of individuals the intestinal injury persists despite gluten removal. Thus, other factors might amplify the ongoing inflammation. Candida albicans is a commensal fungus that is well adapted to the intestinal life without inducing competitive interactions with other microbes or host immune system. However, specific conditions increase Candida pathogenicity. The hypothesis that Candida may be a trigger in CD has been proposed after the observation of similarity between a fungal wall component and two CD-related gliadin T-cell epitopes (Nieuwenhuizen WF et al, Lancet 2003) (Figure 1). However, despite being implicated in intestinal disorders, Candida may also protect against immune pathologies highlighting a more intriguing role in the gut. Previously, we have demonstrated that Candida may behave as a commensal or pathogen in the gut contingent upon several players, including mast cells (MCs) and IL-9 (Renga G et al, Cell reports 2018). Because their ability to disrupt intestinal epithelial barrier and promote inflammation, the MC-IL9 axis may favor Candida pathogenicity eventually contributing to CD pathology. Based on these premises, this project was designed to meet two major aims: first, to define the impact of Candida-MC-IL-9 axis in CD; second, to evaluate whether the manipulation of the inflammatory environment may attenuate the intestinal pathology.
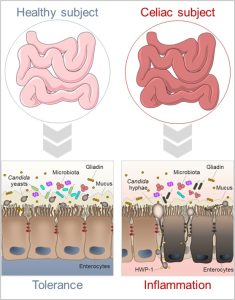
Figure 1 – In the normal digestive tract, Candida albicans lives as a harmless commensal. Inflammatory conditions such as those that occur in celiac disease can influence the commensal-to-pathogen switch of the fungus, likely contributing to the intestinal pathology.
Research plan and results obtained
The research plan has been subdivided into different phases. In the first phase, we have resorted to in vitro preclinical models of CD. First, we tested a potential interaction between gliadin peptides and C. albicans. To this purpose, we cultured Candida with different concentrations of gliadin peptides and we examined the fungal morphology. We found that gliadin peptides were able to promote the formation of large cell-cell aggregates and increase Candida germination. In addition, gliadin-treated Candida cells displayed a higher hyphal length when compared to untreated cells. Next, we investigated the functional effects of Candida and gliadin peptides administration in Caco-2 cells and we found high levels of IL-15, indicating that gliadin peptides have a direct impact on C. albicans morphotype, likely contributing to CD pathogenesis. In the second phase, we resorted to well-defined murine models of CD such as gluten-sensitized and NOD.DQ8 mice. Upon gluten-sensitization, in both models we found that mice infected with C. albicans showed altered intestinal architecture and increased inflammation. Additionally, gluten-sensitized and infected mice showed an increased activation of inflammatory MCs with a strong release of MCPT-1, a chymase that mainly promotes intestinal permeability (Renga G et al, Cell reports 2018). Interestingly, the use of anti-IL-9 mAb ameliorated the intestinal pathology exacerbated by C. albicans, indicating that Candida-MC-IL-9 axis is involved in CD pathogenesis and that IL-9 neutralization may be exploited, to some extent, to prevent intestinal pathology. Another important contributing factor to the pathogenesis of CD is the gut microbiome. A decreased expansion of Lactobacillus spp. and Bifidobacterium (Leonard M et al, Proc Natl Acad Sci U S A 2021) has been associated to an increased pathogenicity of C. albicans. To evaluate whether the modulation of microbiota composition may have a protective activity in CD, we resorted to the microbial metabolite, indole-3-aldehyde (3-IAld), known to play a therapeutic role in mucosal homeostasis and microbial dysbiosis (Zelante T et al, Immunity 2013). We found that 3-IAld was able to control dysbiosis, restrain inflammation and maintain the intestinal barrier integrity through the activation of the AhR/IL-22 axis.
Experimental design and methodologies
The experimental design has been carried out using different approaches:
1) In vitro experiments. C. albicans was cultured in the presence of gliadin peptides and assessed for fungal morphology. Caco-2 cells and mouse bone marrow-derived mast cells were treated with digested gliadin peptides and pulsed with the fungus. Cells were assessed for inflammatory parameters.
2) In vivo experiments. We used two well-defined murine models of gluten sensitivity. Wild-type mice were inbred for 3 generations on a gluten-free diet and challenged with 500 mg/kg gliadin for 4 weeks. After the challenge, mice were infected intragastrically with Candida. In addition, we resorted to NOD AB0 DQ8 (NOD.DQ8) mice that were treated as above and evaluated for inflammatory parameters.
3) Treatments. 3-IAld was administered in cell culture (10 mM) and in mice (18 mg/kg) concomitantly to gluten and Candida administration. The IL-9-neutralizing and isotype control antibodies (10 mg/kg) were administered in concomitance to the infection.
Potential pitfalls and caveats
The pitfalls encountered during this project are related to the lack of an animal model able to properly encompass all features of the disease. Gluten sensitization in gluten-free fed mice represents a valuable biological system to study the local immune responses induced by gluten. However, the breeding of at least three generations of gluten-free fed mice may have a negative effect on the research timeline leading to a delay in the study. The use of NOD.DQ8 mouse model allowed us to solve the issue and determine the pathogenic mechanisms that occur upon gluten ingestion and Candida infection and to evaluate the effect of therapeutic strategies in CD.
Conclusions and discussion
This study has shed light on the environmental factors influencing the commensal-to-pathogen switch of C. albicans in the gut, thus contributing to the intestinal pathology in CD. As primary reservoir of C. albicans, the gut is a perfect habitat where living as a harmless commensal and protecting the host from various microbial insults. However, inflammatory conditions perturbing this peaceful microenvironment have a relevant impact on Candida pathogenicity. MCs and IL-9 are able to finely tune Candida behavior in the gut by promoting commensalism or pathogenicity in a context-dependent manner. Herein, we demonstrated that the Candida-MC-IL-9 axis is involved in CD pathogenesis and contributed to exacerbate the intestinal pathology. We found that IL-9 neutralization and MC modulation counteracted the pathogenic potential of the Candida-IL-9-MC cross-talk eventually contributing to intestinal homeostasis. Given that changes in intestinal microbiota composition and microbial metabolites production are important risk factors in CD, restoring the gut microbiome could help improve intestinal pathology. In this study, we showed that the endogenous metabolite, 3-IAld, ameliorated the inflammatory pathology in murine CD through a primary action on gut mucosal and microbial homeostasis. Specifically, 3-IAld led to the expansion of protective taxa and restored the levels of microbial metabolites such as butyrate, a short-chain fatty acid that enhances intestinal barrier function and mucosal immunity. At a molecular level, 3-IAld was able to activate the host AhR/IL-22 axis, a well-known pathway playing a crucial role in mucosal protection, thus contributing to increase intestinal barrier function while decreasing inflammation in gluten-sensitized mice. Thereby, 3-IAld creates a favorable condition for the reinstatement of Candida commensalism in gluten-sensitized mice.
In conclusion, this study has deciphered some important variables underlying the intriguing role of C. albicans in CD. A better definition of the host-microbiota-C. albicans cross-talk might provide a unique opportunity for develop novel therapeutics that working at the interface between microbes and the host immune system could be used in CD subjects to alleviate pathology and symptoms (Figure 2).
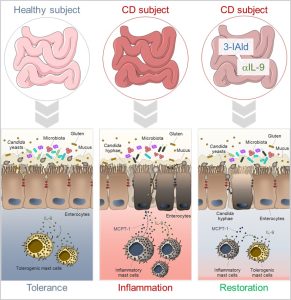
Figure 2 – In celiac disease, inflammatory mast cells (MCs), IL-9 and intestinal dysbiosis create favorable conditions for Candida commensal-to-pathogen transition and the consequent exacerbation of the intestinal pathology. The use of different therapeutic strategies such as indole-3-aldehyde (3-IAld) administration or IL-9 neutralization ameliorates intestinal pathology by controlling microbial dysbiosis, Candida albicans pathogenicity and restraining inflammation.
closed
