closed
Riccardo Troncone, University Federico II, Naples, Italy
3-years Project
Celiac Disease
Area: Immunology
- Grant: FC 053/2013
- Title: Natural History of Innate Immune Activation in Celiac Disease: triggers, pathways and clinical implications
- Topic: Immunology of Celiac Disease
- Duration: Triennial Project
- Principal Investigator: Riccardo Troncone, University Federico II, Naples
Publications originating from the Project:
- Setty M, Discepolo V, Abadie V, Kamhawi S, Mayassi T, Kent A, Ciszewski C, Maglio M, Kistner E, Bhagat G, Semrad C, Kupfer SS, Green PH, Guandalini S, Troncone R, Murray JA, Turner JR, Jabri B. Distinct and synergistic contributions of epithelial stress and adaptive immunity to functions of intraepithelial killer cells and active celiac disease. Gastroenterology 2015;149:681-91. https://pubmed.ncbi.nlm.nih.gov/26001928/
- Tosco A, Maglio M, Paparo F, Greco L, Troncone R, Auricchio R. Discriminant score for celiac disease based on immunohistochemical analysis of duodenal biopsies. J Pediatr Gastroenterol Nutr. 2015;60:621-5. https://pubmed.ncbi.nlm.nih.gov/25514620/
- Borrelli M, Gianfrani C, Lania G, Aitoro R, Ferrara K, Nanayakkara M, Ponticelli D, Zanzi D, Discepolo V, Vitale S, Barone MV, Troncone R, Auricchio R, Maglio M. In the Intestinal Mucosa of Children With Potential Celiac Disease IL-21 and IL-17A are Less Expressed than in the Active Disease. Am J Gastroenterol. 2016;111:134-44. https://pubmed.ncbi.nlm.nih.gov/26753888/
- Paolella G, Lepretti M, Barone MV, Nanayakkara M, Di Zenzo M, Sblattero D, Auricchio S, Esposito C, Caputo I. Celiac anti-type 2 transglutaminase antibodies induce differential effects in fibroblasts from celiac disease patients and from healthy subjects. Amino Acids. 2017;49:541-550. https://pubmed.ncbi.nlm.nih.gov/27613408/
- Camarca A, Auricchio R, Picascia S, Fierro O, Maglio M, Miele E, Malamisura B, Greco L, Troncone R, Gianfrani C. Gliadin-reactive T cells in Italian children from preventCD cohort at high risk of celiac disease. Pediatr Allergy Immunol 2017;28:362-369. https://pubmed.ncbi.nlm.nih.gov/28339124/
- Nanayakkara M, Lania G, Maglio M, Auricchio R, De Musis C, Discepolo V, Miele E, Jabri B, Troncone R, Auricchio S, Barone MV. P31-43, an undigested gliadin peptide, mimics and enhances the innate immune response to viruses and interferes with endocytic trafficking: a role in celiac 6. disease. Sci Rep. 2018 Jul 17;8(1):10821. https://pubmed.ncbi.nlm.nih.gov/30018339/
- Passannanti F, Nigro F, Gallo M, Tornatore F, Frasso A, Saccone G, Budelli A, Barone MV, Nigro R. In vitro dynamic model simulating the digestive tract of 6-month-old infants. PLoS One. 2017 Dec 20;12(12):e0189807. https://pubmed.ncbi.nlm.nih.gov/29261742/
- Paolella G, Lepretti M, Martucciello S, Nanayakkara M, Auricchio S, Esposito C, Barone MV, Caputo I. The toxic alpha-gliadin peptide 31-43 enters cells without a surface membrane receptor. Cell Biol Int. 2018 Jan;42(1):112-120. https://pubmed.ncbi.nlm.nih.gov/28914468/
The Study:
Project rationale and aims
The reciprocal contribution of gliadin and viral infections and/or other triggers to induce CD is unknown The main aim of our proposal was to study InIR pathways, activated by either gluten peptides and/or viruses, in the context of different stages of CD. We have focused on the pathways activated by viral ligands and gliadin peptides and how they co-operated to activate the INF-alpha and NFkB pathways. Moreover we have studied the possible mechanisms of activation of these pathways by gliadin peptides. We have also focused on potential CD (a pre-CD condition) in order to identify markers that could help predicting the development of CD. Identifying such markers could allow implementing prevention strategies for subjects showing a high risk profile to future CD development.
Aim 1. We aimed to investigate the expression of IL-15, Type-1 IFN and its downstream effector MxA, a protein induced upon viral infections. We analyzed the expression of those markers in the small intestinal biopsies of treated as well as untreated CD patients, compared to controls with and without intestinal inflammation.
Aim 2. Gliadin is the main environmental factor involved in CD development; however it is now accepted that other environmental triggers may play a role in disease pathogenesis. Among those, viral infections have been proposed as triggers of CD in genetically susceptible individuals. The main objective of this aim was to investigate the ability of gliadin peptides, in particular P31-43, and viruses, through the use of viral ligands, to induce innate immune activation in the context of CD. We investigated the effects of the toxic gliadin peptide P31-43 and the TLR-7 ligand Loxoribine (7-allyl-7,8-dihydro-8-oxo-guanosine, LOX) a viral ligand acting on the MYD88-dependent TLR7 signaling pathway (Heil F. et al. Eur J Immunol 2003), and dissected the molecular pathways activated by these stimuli.
Aim 3. To demonstrate the role of the delay of vesicular trafficking in the activation of IFN-alpha, we induced a delay in the maturation of endocytic trafficking by silencing HRS, a master regulator of endocytic maturation. HRS silencing mimicked all the measured effects of P31-43 on the TLR7 pathway: the delay of TLR7 in the early vesicles; increase in the MyD88/TLR7 complex and proteins; and the increase in IFN-alpha 7/17 mRNA, MxA protein and NF-κB activation.
Research plan and results obtained
We demonstrated an up-regulation of the type-1 IFN pathway in CD patients (Figure 1).
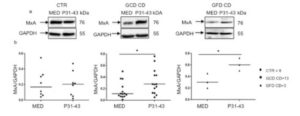
Figure 1. P31-43 enhances MXA expression in GCD and GFD CD biopsies. (a) Western blot analysis of protein lysates from small intestinal biopsies of CD patients at GCD, GFD and controls cultivated for 24 h with and without P31-43. The upper line was blotted with anti-MXA and the second line was blotted with anti-GADPH antibodies as loading control. (b) Densitometric analysis. The relative amounts of MXA were normalized to GADPH. Horizontal lines represent the median. Student t test respect to the control (CTR) samples. * = p < 0.05. One-way Anova test, Bonferroni corrected.
Afterwords we wanted to test the ability of different environmental factors, in particular gliadin and viral ligands to activate the type-1 IFN pathway in intestinal epithelial cells. In particular we tested the effect of the toxic gliadin peptide P31-43 and the TLR7 agonist LOX on CaCo2 cells and dissected the mechanism through which these stimuli activate innate immunity showing that:
1) P31-43 and LOX activate type-1IFN innate immune pathway and promote inflammation, inducing NF-kB up-regulation in a MyD88 dependent way.
2) MyD88 and TLR7 can cooperate to activate the downstream signaling that finally leads to the up-regulation of the type-1 IFN and the NFkB pathway.
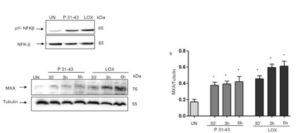
Figure 2. P31-43 like LOX induced markers of inflammation and of innate immunity activation.
P31-43 like viral ligand LOX activated NFk-B and INF- pathways. A. Western blot analysis of protein lysates from CaCo-2 cells treated as indicated for 30’. The upper line represent the phosphorylated form of NFKβ (pNFKβ) and the second line the total NFKβ protein. B. (a) Western blot analysis of protein lysates from CaCo-2 cells treated as indicated. The upper line was blotted with anti-MXA and second line was blotted with anti-tubulin antibodies used as loading control. One representative of 3 independent experiments. (b) Densitometric analysis. The relative amounts of MXA were normalized to tubulin. Columns represent the mean and bars the standard deviation of 3 independent experiments.* p<0.05, **p<0.01, ***p<0.01. One-way Anova test, Bonferroni corrected vs Untreated (UN).
3) P31-43 and Lox can activate synergistically the MyD88-TLR7. Suboptimal concentrations of P31-43 and Lox enhanced type-1 IFN pathway (Fig. 3) both at a transcriptional (IFN-alpha mRNA) and translational level (MxA protein). These results reinforced the hypothesis that gliadin and viral ligands could synergize to promote innate immune responses in intestinal epithelial cells.

Figure 3. Suboptimal concentrations of P31-43 and Lox together induced MXA and INF- expression. Western blot analysis of protein lysates from CaCo-2 cells treated overnight with suboptimal concentrations of P31-43 (20 micrograms) and LOX (250 microM) (a). Anti-MxA Ab (upper panel) and anti-tubulin Ab (lower panel) as a loading control have been used. One representative blot out of 3 independent experiments. (b) Densitometric analysis. The relative amounts of MXA were normalized to tubulin. Columns and bars represent mean and standard deviation of 3 independent experiments. *p<0.05 vs untreated (UN). One-way Anova test, Bonferroni corrected. (c) Quantitative PCR analysis of INF-alpha 17 mRNA in CaCo2 cells after treatment with P31-43 and Lox at suboptimal concentration. Student-t Test vs untreated ***p< 0.01
4) Delaying the maturation from early to late vesicles activate the TLR7 pathway.inducing a delay in the maturation of the vesicles by silencing HRS protein (si-HRS) and analyzing the trafficking of TLR7 in the early and late compartments. Under these conditions, we found that, similar to the effects of the gliadin peptide P31-43 and LOX, the amount of TLR7 in complexes with MyD88 increased in a statistically significant manner relative to the untreated sample (p = 0.03). MxA (p = 0 .02), IFN-α 7 (p = 0.0009) and 17 (p = 0.04) mRNAs and the phosphorylated form of NF-κB (p =0.03) were increased after HRS silencing (Figure 4). In conclusion, the alteration of the vesicular trafficking induced by si-HRS could activate the TLR7 pathway and the IFN-α mediated immune response together with the NF-κB-mediated inflammation.
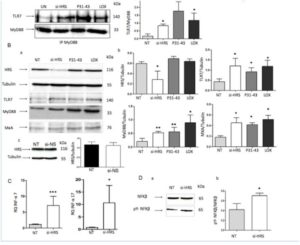
Figure 4: Delaying maturation of endocytosis using si-HRS activates the TLR7 pathway in CaCo-2 cells. A. Silencing of HRS as well as treatment for 30’ with P31-43 or the viral ligand LOX induced an increase in the MyD88/TLR7 complex. a) Western blot analysis of immunoprecipitated MyD88 in CaCo-2 cells. The upper line was stained for TLR7, and the second line was stained for MyD88. The treatments are indicated. The results shown are representative of 3 independent experiments. b) Densitometric analysis. The relative amounts of TLR7 in the complex were normalized to the immunoprecipitated MyD88 levels. The treatments are indicated. The columns represent the means, and the bars represent the standard deviations of 3 independent experiments. NT = untreated. Student’s t-test, compared with the untreated NT sample. *: p < 0.05. B. Silencing of HRS or treatment with P31-43 or the viral ligand LOX for 30’ increased the expression of TLR7, MyD88 and MxA. a) Western blot analysis of protein lysates from CaCo-2 cells that had been treated as indicated. The upper line was blotted with anti-HRS, and second line was blotted with anti-tubulin antibodies as the loading control. The third, fourth and fifth lines were blotted with antibodies to TLR7, MyD88 and MxA. The results shown are representative of 3 independent experiments. (b) Densitometric analysis. The relative amounts of HRS, TLR7, MyD88 and MxA were normalized to that of tubulin. The treatments are indicated. The columns represent the means, and the bars represent the standard deviations of 3 independent experiments. NT = untreated. Student’s t-test, compared with the NT sample. *: p < 0.05, **: p < 0.01. c) Control non-specific silencing of mRNA was ineffective on HRS expression. Western blot analysis of HRS protein before and after silencing with non-specific silencing of mRNA (si-NS). The upper line was blotted with anti-HRS, and the second line was blotted with anti-tubulin antibodies. The results shown are representative of 3 independent experiments. Densitometric analysis. The relative amounts of HRS were normalized to those of tubulin. The columns represent the means, and the bars represent the standard deviations of 3 independent experiments. NT = untreated. Student’s t-test. C. Increased IFN-α 7 and 17 mRNA in CaCo-2 cells transfected with si-HRS mRNA. RQ = relative quantities of IFN-α 7 and 17 mRNA. The columns represent the means, and the bars represent the standard deviations of 3 independent experiments. NT = untreated. Student’s t-test compared with the NT sample, *: p < 0.05, ***: p < 0.001. D. Silencing HRS induced an increase in the levels of pY-NF-κB. (a) Western blot analysis of protein lysates from CaCo-2 cells that had been treated as indicated. The lower line represents the phosphorylated form of NF-κB (pY-NF-κB), and the upper line represents the total NF-κB protein. The results shown are representative of 3 independent experiments. (b) Densitometric analysis. The relative amounts of pY-NF-κB were normalized to the total NF-κB levels. The columns represent the means, and the bars represent the standard deviations of 3 independent experiments. NT = untreated. Student’s t-test compared with the NT sample, *: p < 0.05.
Experimental design and methodologies
Duodenal biopsies have been collected from at least 20 active celiac disease (CD) and at least 20 non-celiac control subjects with (CTR GI) and without (CTR) gastrointestinal issues. We included also at risk subjects with normal intestinal duodenal biopsy, including first-degree family members of CD patients and potential CD patients. Finally we recruited CD patients on a gluten-free diet (GFD) for at least 2 years to test whether the observed changes could be related to gluten consumption. Biopsy specimen have been included in paraffin or in OCT for later IHC; extra specimen have been stored in RNA-later of in lysis buffer for later PCR and Western blot (WB) analysis respectively. RNA was isolated from duodenal biopsies stored in RNA-later and Real-time qPCR was performed to test for IFN-a, IFN-b, Mx1, IL15Ralpha and IL-21. Western blot analysis was performed on lysates from duodenal biopsies. Immunohistochemistry (IHC) was performed on formalin fixed paraffin embedded duodenal sections to test for IL-15 and MxA, while frozen sections have been used to test for IL-21 expression. ELISA has been performed on culture supernatants to assess IL-21 levels in organ culture of duodenal biopsies from active patients and controls. Immunofluorescence and confocal analysis allowed to study the endocytic pathway
Potential pitfalls and caveats
We did not encounter any difficulties in the project.
Conclusions
The data reported here imply:
1) innate immunity plays an important role in CD. The activation of innate immunity is indicated by both signs of epithelial stress and involvement of special cell subsets (NK, gammadelta)
2) Epithelial stress and activation of innate immunity (IL15 and MXA) can be induced by an undigested gliadin peptide, P31-43.
3) Gliadin peptide P31-43 can cooperate with viral ligands to induce epithelial stress and MXA/INF-alpha. Vesicular trafficking is involved in P31-43 effects
4) Innate immunity is activated also in Potential patients. But the role of this activation is still under investigation
The results presented here suggest that together with viral infections alimentary proteins, able to mimic and potentiate the innate immune response to viruses can trigger an autoimmune disease. In addition to CD, other autoimmune diseases are the result of the interactions of several factors, including genetics and the environment. Type 1 interferon activation is linked to autoimmunity not only in CD but also in several other human diseases, including type 1 diabetes. In some of these diseases, unlike CD, genetic polymorphisms that directly activate the type 1 interferon pathways are present, although these alterations are not by themselves sufficient to induce the disease. The penetrance of such genetic profiles is determined by mostly unknown environmental factors, including disturbances in the microbiota and/or frequent viral infections. In this study, we showed that another environmental factor could be an alimentary protein such as gliadin. These results suggested that gluten itself could be a risk factor under these conditions.
