closed
Carmen Gianfrani, Istituto di Biochimica e Biologia Cellulare - Consiglio Nazionale delle Ricerche, Naples, Italy
2-years Project
Celiac disease
Area: Immunology
Grant: 002/2020
- Title: To explore the role of IL4+ and TCRγδ+ cells as markers of mucosa damage in celiac disease.
- Duration: 2 Years Project
- Principal Investigator: Carmen Gianfrani, Istituto di Biochimica e Biologia – CNR, Naples, Italy
- Collaboration: Dipartimento di Scienze Mediche Traslazionali – Sezione di Pediatria, Università Federico II di Napoli, Naples, Italy
Publications originating from the Project
- manuscript in preparation
THE STUDY
Project rationale and aims
In childhood, celiac disease (CeD) manifests with two main forms: acute-CeD and potential-CeD. Both forms are characterized by positivity of HLA DQ2/DQ8 risk alleles and of anti-tissue transglutaminase antibodies (anti-tTG), but differ for the lesion entity of intestinal mucosa. We demonstrated in previous studies that the intestinal mucosa of potential-CeD is characterized by a marked infiltration of T cells releasing interleukin-4 (IL4) and a very low number of TCRγδ+ T cells. By contrast, in the gut mucosa of acute-CeD with villous atrophy, we observed an opposite scenario, as TCRγδ+ T cells were markedly expanded whilst IL4-secreting T cells were almost undetectable. These findings suggested an involvement of these two cell subsets in the transition from potential to acute disease, and a protective role of IL4 in preventing the villous atrophy. The aim of this project was to investigate the regulatory mechanisms through which IL4 counteracts the inflammatory response to gluten in the intestinal mucosa of CeD patients. Short-term T-cell lines reactive to gliadin (st-TCLs) were in vitro generated from celiac gut biopsies using exogenous IL4 as growth factor. Changes on phenotype of cell infiltrates, profile of cytokines produced and specificity to immunodominant gliadin peptides were evaluated compared with control st-TCLs obtained in the absence of IL4. More specifically, we analyzed if IL4 treatment affected the expansion of effector CD4+ and CD8+ T-cell subsets, of TCRγδ+ T cells, as well as of regulatory Tr1 and Foxp3+ T cells. Alteration of gliadin recognition pattern was assessed by measuring IFN-γ production by ELISA and flow cytometry.
Research plan and results obtained
Intestinal biopsies were obtained from 20 pediatric patients, 10 with potential-CeD (mean age and range: 7 years; 2.3-17.9) and 10 with acute-CeD (8.1 years; 3.3-16.6) and processed to generate gliadin-specific T-cell lines with or without exogenous IL4 as described below in the experimental design and methodologies paragraph. Cell culture supernatants were collected at different time points, starting from 1 week after the cell culture set-up and up to 5 weeks of culture, more precisely at day 8, 16, 19, 23, 26, 30 and 33 to evaluate the level of IFN-γ and IL10 released that were measured by ELISA. IFN-γ release resulted markedly reduced in supernatants of IL4-TCLs compared to control TCLs in both potential- and acute-CeD patients. However, the most significant reduction was observed in children with potential disease (Figure 1, panel A&B).
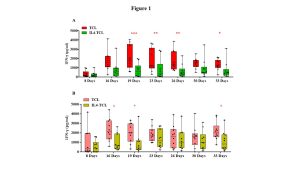
Figure 1: IL4 treatment reduces the IFN-γ production in culture supernatants of intestinal T-cell lines. The st-TCLs generated from intestinal biopsies of 10 children with potential-CeD and 10 children with acute-CeD were analyzed for the amount of IFN-γ released. The supernatants of control- and IL4-TCL were collected at different days of culture (8, 16, 19, 23, 26, 30, and 33). IFN-γ concentration was measured by ELISA and showed as pg/ml. The bars represent median and range (95th percentile). Statistical analysis was performed with Student’s paired T-test and GraphPad Prism software, with * p< 0.05, ** p ≤ 0.01. Panel A: IFN-γ production measured in potential-CeD patients. Panel B: IFN-γ production measured in acute-CeD patients.
The cell culture supernatants were also analyzed at the same time points for the IL10 release, a cytokine with important regulatory function in the gut mucosa. A slight increase, not statistically significant, of IL10 production was observed in IL4-TCLs from potential-CeD patients at day 26 and 30, compared with control TCLs (Figure 2, panel A). In acute-CeD children, no differences in IL10 production were observed in culture supernatants between IL4- and control-TCL at any time points analyzed (Figure 2, panel B).
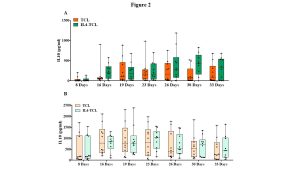
Figure 2: IL4 treatment had no effect in the release of IL10 in culture supernatants of intestinal T-cell lines. The st-TCLs generated from intestinal biopsies of 10 children with potential-CeD and 10 children with acute-CeD were analyzed for the amount of IL10 released. The supernatants of control- and IL4-TCL were collected at different days of culture (8, 16, 19, 23, 26, 30, and 33). IL10 concentration was measured by ELISA and showed as pg/ml. The bars represent median and range (95th percentile). Statistical analysis was performed with Student’s paired T-test and GraphPad Prism software, with * p< 0.05, ** p≤ 0.01. Panel A: IL10 production measured in potential-CeD patients. Panel B: IL10 production measured in acute-CeD patients.
To better investigate the role of IL4 on the activation/expansion of gliadin-reactive T lymphocytes, we performed functional specificity tests. Overall, 38 T-cell lines were analyzed for gliadin specificity, of them 18 (9 control-TCL and 9 IL4-TCL) were obtained from potential-CeD patients and 20 (10 control-TCL and 10 IL4-TCL) were generated from acute-CeD patients. Cells were stimulated with autologous or HLA-matched immortalized B-cells and with a deamidated peptic-tryptic digest of gliadin (tTG-PT). Furthermore, in order to evaluate whether the IL4 effect is mediated by the activation of regulatory pathway, the responsiveness of T cells to gliadin was also assessed in the presence of antibodies neutralizing IL10 receptor and TGFβ. The activation of gliadin-specific T cells was assessed by detecting IFN-γ production by ELISA after 48 hours of stimulation. As shown in Figure 3, panel A, IL4 in vitro treatment resulted in a slight reduction of IFN-γ production in children with potential-CeD. No substantial differences were observed when IL10 and TGFβ were neutralized. Surprisingly, in TCL generated from children with acute-CeD, IL4 treatment resulted in a slight, not statistically significant increase of IFN-γ production (Figure 3, panel B).
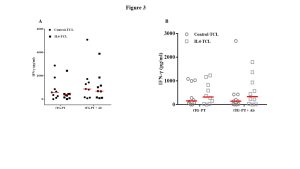
Figure 3: IL4 treatment induced an inhibition of IFN-γ production by intestinal T cells in response to gliadin. Data are shown as IFN-γ production by each control-TCL and IL4-TCL established from the same potential patient in response to both deamidated gliadin digest (tTG-PT). The functional assays were run out also in presence or absence of anti-IL10R and anti-TGFβ neutralizing antibodies (Ab). The data showed are the pg/ml detected in each TCL. Red lines represent the median values, statistical analysis was performed using paired Student’s T-test by GraphPad Prism Software. Panel A: IFN-γ production in response to gliadin measured in potential-CeD patients. Panel B: IFN-γ production in response to gliadin measured in acute-CeD patients.
In order to analyze the effect of IL4 on the in vitro expansion of different intestinal cell subsets, we analysed by multiparametric flow cytometry, the percentage of CD4+, CD8+, TCRγδ+ T cells in both control- and IL4-treated TCLs generated from 9 potential-CeD (Figure 4, panel A) and 10 acute-CeD patients (Figure 4, panel B). As shown in Figure 4A, exogenous IL4 induced a statistically significant expansion of CD4+ T cells and significantly reduced the frequencies of CD8+ T and TCRγδ+ T cells. By contrast, IL4 has not effects on the expansion of the different T cell subsets in TCLs generated from children with villous atrophy, as similar percentages of CD3+, CD4+, CD8+ and TCRγδ+ T cells were observed between control- and IL4-TCLs (Figure 4B).

Figure 4: The invitro treatment with IL4 inhibits the expansion of CD8+ and TCR-γδ+ T cells mainly in children with potential-CeD. Representative flow cytometry dot-plots of control-TCL and IL4-TCL generated from potential-CeD patients (Panel A) and acute-CeD patients (Panel B) are reported for each cell population analyzed (CD4+, CD8+, TCR-γδ+ T cells).
Experimental design and methodologies
Intestinal mucosa explants were digested in vitro with collagenase-A to disrupt the tissue matrix. Thereafter, cells were in vitro stimulated with irradiated autologous PBMC and gliadin enzymatic digest with or without exogenous IL4 added as growth factor (respectively indicated as IL4-TCL and control-TCL). Cytokine productions by mucosa lymphocytes were evaluated by ELISA either in cell supernatants collected at different time points (day 8, 16, 19, 23, 26, 30 and 33) and in response to gliadin in short-term T-cell lines after 48 hours of antigen incubation. Phenotypic changes in control-TCLs and IL4-TCLs were assessed by multiparametric flow cytometry (Canto II and LSR2 flow cytometers, supplied with FACSDiva software).
Children were enrolled at the Department of Translational Medical Sciences, Section of Pediatrics, University Federico II of Naples and had an esophago-gastro-duodenoscopy (EGDS) for suspicion of celiac disease. Children with a diagnosis of acute or potential celiac disease were at the time of enrolment all positive for the anti-tissue transglutaminase antibodies-IgA (cut-off for positive serology > 7 or 9 U/mL if assessed by immunoenzymatic assay, or >30 U/mL if assessed by chemiluminescent immunoassay). Figure 5 schematically illustrates the experimental design and methodologies.
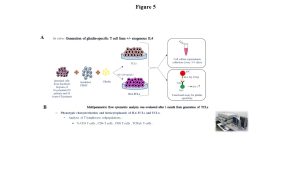
Figure 5: Experimental design used for the generation of short-term TCLs from celiac intestinal biopsies to assess IL4 regulatory function. Panel A: Generation of gliadin-specific, short-term T-cell lines (TCL) with and without exogenous IL4 and functional antigen specificity assays. Panel B: Multiparametric flow cytometry analysis to assess IL4-mediated changes in T-cell subsets phenotype.
Potential pitfalls and caveats
The possible pitfall, already hypothesized in the proposal, was the low number of biopsies taken during the EGDS in the young children enrolled in the study that limited the intestinal cell recovery required for the ex vivo cell phenotype analysis. To overcome this limitation, we planned to generate short-term T-cell lines from biopsies. We have enrolled overall 20 paediatric patients and generated 40 T-cell lines (20 control-TCLs and 20 IL4-TCLs), all of them analyzed to evaluate the inhibitory role of IL4 on intestinal immune response against gliadin.
Conclusions and discussion
In recent years, scientific research is focused on the possibility to identify categories of individuals at risk of becoming celiac, in order to anticipate diagnosis in such individuals before the disease manifests clinically. In pediatric patients first diagnosed with potential celiac disease, it has been observed, that about one-third develop intestinal atrophy during a 9-year observation period on a free diet, but the mechanisms underlying the evolution of mucosal damage and the transition from the potential to the acute phase are still unclear. Our work demonstrates a previously unexplored immunoregulatory function of IL4 in celiac disease and in particular in the potential-CeD form, supporting the hypothesis that there are peculiar differences between the two forms of the disease and that IL4 might have a role in preventing villous atrophy. The reduction of INF-γ production, in cell culture supernatants and in response to deamidated gliadin, associated with a significant increase of CD4+ T cells and a reduction of CD8+ T cells and of TCR-γδ+ T cells suggested an anti-inflammatory role of this cytokine in the intestinal mucosa of patients with potential-CeD.
